Last Updated on 19 November 2025 by editablematerials.in
CBSE Class 10 Science Chapter 1 Case Study Questions – based on Chemical Reactions and Equations – help students understand how substances interact to form new products. This chapter explains how to write chemical equations, balance them, identify different types of chemical reactions, and observe real-life examples of chemical changes.
The questions below follow the CBSE Class 10 competency-based format and focus on application, reasoning, and real-life problem-solving skills.
🔍 Chapter Summary
Chapter 1 – Chemical Reactions and Equations introduces the concept of chemical changes where new substances with new properties are formed. Students learn to represent reactions using word equations and balanced chemical equations to satisfy the law of conservation of mass. The chapter also covers different types of chemical reactions such as combination, decomposition, displacement, double displacement, oxidation, and reduction.
The chapter also highlights important real-life processes like rusting of iron and rancidity of food, and explains methods to prevent these changes. Through activities and observations, students understand how to identify chemical changes based on indicators like heat, gas formation, colour change, and precipitate formation.
📝 Case Study Question 1
Read the passage and answer the questions:
In a school laboratory, the teacher heated a small ribbon of magnesium in the flame of a burner. The ribbon burned with a dazzling white flame and a white powder was collected in a watch glass. The class was told that this white powder is magnesium oxide. Later, the teacher wrote the word equation and then the chemical equation on the board and discussed the idea of balanced and unbalanced equations.

Answer the following:
- Write the word equation for the reaction between magnesium and oxygen.
- Write the balanced chemical equation for this reaction using symbols and formulae.
- Is this reaction a combination reaction or a decomposition reaction? Give a reason.
- State any one observable change in this activity that indicates a chemical reaction has taken place.
Answers:
- Word equation: Magnesium + Oxygen → Magnesium oxide
- Balanced chemical equation: 2Mg + O2 → 2MgO
- It is a combination reaction because two substances (magnesium and oxygen) combine to form a single product, magnesium oxide.
- The magnesium ribbon burns with a dazzling white flame and a new white powder (magnesium oxide) is formed, indicating a chemical reaction.
🔗 White Label Editable Study Material – Download & Customize
🎓 Looking for fully editable Study Materials with your own branding or watermark?
✅ Ready to use in class
✅ Fully customizable
✅ White Label Materials
✅ Available in Word (.docx) or PageMaker format
✅ Add your school or institute logo
👉 Download CBSE Class 10 Editable Materials
📝 Case Study Question 2
Read the passage and answer the questions:
In a laboratory activity, students added dilute sulphuric acid to zinc granules in a test tube. They observed brisk effervescence and a gas was evolved that burned with a pop sound when a burning matchstick was brought near it. The temperature of the test tube increased on touching it. The teacher explained that a salt called zinc sulphate and hydrogen gas were formed.
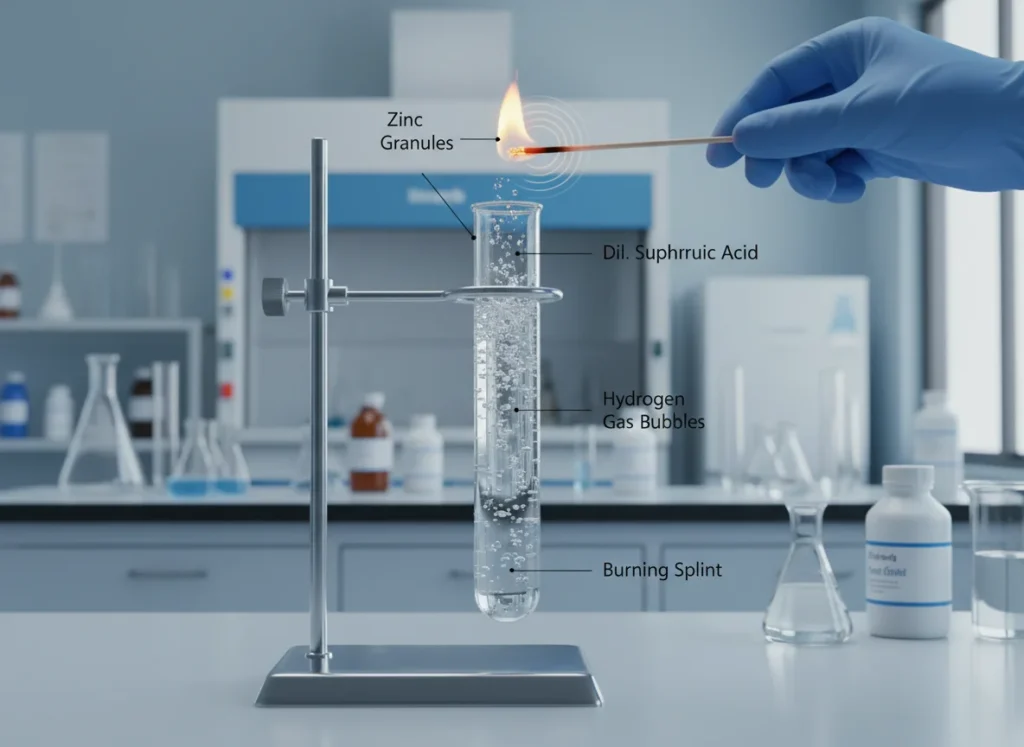
Answer the following:
- Write the balanced chemical equation for the reaction between zinc and dilute sulphuric acid.
- Which gas is produced in this reaction and how is it tested?
- Is this reaction exothermic or endothermic? Give a reason based on the observation.
- Which type of reaction is this: displacement or double displacement? Justify your answer.
Answers:
- The balanced chemical equation is: Zn + H2SO4 → ZnSO4 + H2↑
- The gas produced is hydrogen. It is tested by bringing a burning matchstick near it; the gas burns with a characteristic ‘pop’ sound.
- The reaction is exothermic because the test tube becomes warm, showing that heat is released during the reaction.
- It is a displacement reaction because zinc displaces hydrogen from dilute sulphuric acid to form zinc sulphate and hydrogen gas.
📝 Case Study Question 3
Read the passage and answer the questions:
A house owner noticed that the iron gate of the house and the iron grills near the garden had developed a reddish-brown, flaky coating after the rainy season. The mechanic told him that the iron has undergone rusting due to the presence of moisture and air. To prevent further damage, the owner decided to get the gate painted with anti-rust paint. In another case, a packet of chips mentioned “Packed in nitrogen” to keep it fresh and crisp.

Answer the following:
- What is the name of the process due to which the iron gate gets a reddish-brown coating? Write its common name and define it.
- Name the two essential conditions required for this process to occur on iron.
- Why is painting the iron gate a useful method to prevent this process?
- Why are food packets like chips often flushed with nitrogen gas? Name the undesirable change in food that is being prevented.
Answers:
- The process is called corrosion. In the case of iron, it is commonly called rusting. Corrosion is the gradual eating away of metals when they are attacked by substances around them such as moisture, air, acids, etc.
- The two essential conditions for rusting of iron are the presence of moisture (water) and air (oxygen).
- Painting forms a protective layer on the iron surface and prevents contact of iron with air and moisture, thereby preventing rusting.
- Food packets like chips are flushed with nitrogen to prevent oxidation of fats and oils present in the food. This prevents rancidity, which would otherwise spoil the taste and smell of the food.
🛠️ Download PPT for CBSE Class 10
Looking for ready-to-use, visually engaging PowerPoint presentations for CBSE Class 10?
We offer chapterwise editable PPTs designed according to the latest CBSE Class 10 syllabus. Perfect for classroom teaching, smart board use, or online lessons.
✅ Features of Our Class 10 PPTs:
- Suitable for offline or online teaching platforms
- Covers all subjects: Science, Maths, SST, English, and Hindi
- Fully editable in PowerPoint (.pptx format)
- Includes diagrams, key points, and animations
- Easy to customize with school logos or watermarks
🎓 Who Is It For?
- CBSE school teachers
- Coaching institutes
- Tutors and academic content creators
🔗 Download Now
👉 Download CBSE Class 10 PPTs (Editable Format)
More Case Study Questions from Class 10 Science
- CBSE Class 10 Science Chapter 1 Case Study Questions with Answers – Chemical Reactions and Equations
You may also like

❓ Frequently Asked Questions (FAQs)
Q1: Are these questions based on the latest CBSE Class 10 Science syllabus?
A1: Yes, all questions follow the latest NCERT syllabus and CBSE competency-based question format.
Q2: Can I use these questions for class assignments or tests?
A2: Absolutely! These case studies are ideal for worksheets, oral discussions, or written tests.
Q3: Are editable versions of these questions available?
A3: Yes. We offer fully editable Word (.docx) versions of all case study and assertion-reason questions. You can add your school logo, watermark, or modify content to suit your teaching needs.
👉 Get Editable Materials Here
Q4: Can I edit and customize these questions for my school or coaching center?
A4: Yes. Download our DOCX version, add your logo, edit text, and use them your way!
Q5: Do you provide questions for other chapters too?
A5: Yes, we’re uploading chapterwise case study questions for Classes 6 to 12.
Q6: What are the best websites for my CBSE Preparation?
A6: Some of the best website for CBSE is Physics Gurukul, Xam Content and Success Router. They are having a large collection of CBSE resources.


